Embark on an academic journey with our in-depth exploration of Unit Chemical Reactions Balancing Equations WS 2 Answer Key. This comprehensive guide unravels the intricacies of stoichiometry, empowering you with the knowledge to decipher and solve chemical equations with precision.
Delve into the fundamental concepts of balancing equations, discover innovative methods for redox reactions, and engage in hands-on practice to solidify your understanding. Prepare to conquer the complexities of chemical reactions and unlock the secrets of stoichiometric calculations.
Unit Chemical Reactions Balancing Equations
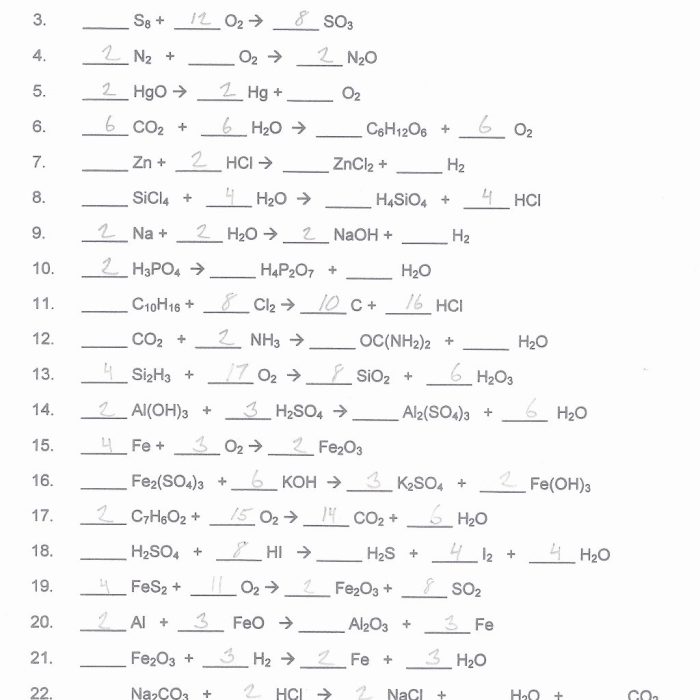
In chemistry, a chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Balancing chemical equations is a crucial aspect of understanding chemical reactions and their quantitative relationships.
A balanced chemical equation ensures that the number of atoms of each element on the reactants’ side of the equation equals the number of atoms of the same element on the products’ side. This principle, known as the law of conservation of mass, is fundamental to stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions.
Methods for Balancing Chemical Equations
There are several methods for balancing chemical equations, each with its own advantages and limitations.
Half-Reaction Method
The half-reaction method is commonly used to balance redox reactions, which involve the transfer of electrons between reactants. In this method, the reaction is divided into two half-reactions, one for oxidation and one for reduction. Each half-reaction is then balanced separately before combining them to form the overall balanced equation.
Oxidation Number Method
The oxidation number method is another approach to balancing redox reactions. It involves assigning oxidation numbers to each atom in the reactants and products and using these numbers to determine the number of electrons transferred. The equation is then balanced by adjusting the coefficients of the reactants and products to ensure that the total change in oxidation numbers is zero.
Both the half-reaction method and the oxidation number method can be used to balance redox reactions. The choice of method depends on the complexity of the reaction and the preferences of the individual.
Practice Balancing Equations, Unit chemical reactions balancing equations ws 2 answer key
Balancing chemical equations is a skill that requires practice. The following table provides a set of unbalanced chemical equations for students to practice balancing:
| Unbalanced Equation | Balanced Equation | Explanation |
|---|---|---|
| 2H2 + O2 → H2O | 2H2 + O2 → 2H2O | Coefficients adjusted to balance the number of hydrogen and oxygen atoms. |
| Fe + HCl → FeCl2 + H2 | Fe + 2HCl → FeCl2 + H2 | Coefficient of HCl adjusted to balance the number of chlorine atoms. |
| CH4 + 2O2 → CO2 + 2H2O | CH4 + 2O2 → CO2 + 2H2O | Coefficients adjusted to balance the number of carbon, hydrogen, and oxygen atoms. |
Applications of Balanced Chemical Equations
Balanced chemical equations have numerous applications in chemistry and beyond:
- Stoichiometry:Balanced equations allow us to calculate the stoichiometric ratios of reactants and products, which are essential for predicting the quantities of reactants and products involved in a reaction.
- Chemical Engineering:Balanced equations are used in the design and optimization of chemical processes, ensuring efficient use of resources and minimizing waste.
- Industrial Processes:Balanced equations are crucial for determining the optimal conditions for industrial chemical reactions, such as temperature, pressure, and catalyst requirements.
Expert Answers: Unit Chemical Reactions Balancing Equations Ws 2 Answer Key
What is the significance of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side. This adherence to the law of conservation of mass is crucial for accurate stoichiometric calculations.
Can you explain the half-reaction method for balancing redox reactions?
The half-reaction method involves dividing the redox reaction into two half-reactions, one for oxidation and one for reduction. Each half-reaction is then balanced separately before combining them to form the overall balanced equation.
What are the applications of balanced chemical equations in real-world scenarios?
Balanced chemical equations find widespread applications in chemical engineering and industrial processes. They enable the prediction of product quantities, optimization of reaction conditions, and the design of efficient chemical reactors.