What is the major product of the reaction shown below – Delving into the intricacies of chemical reactions, this article unravels the major product of the reaction shown below, providing a comprehensive understanding of its chemical structure, functional groups, and the underlying reaction mechanism. This exploration will shed light on the significance of the reaction and its diverse applications, unveiling its importance in both industrial and academic realms.
What is the Major Product of the Reaction Shown Below?
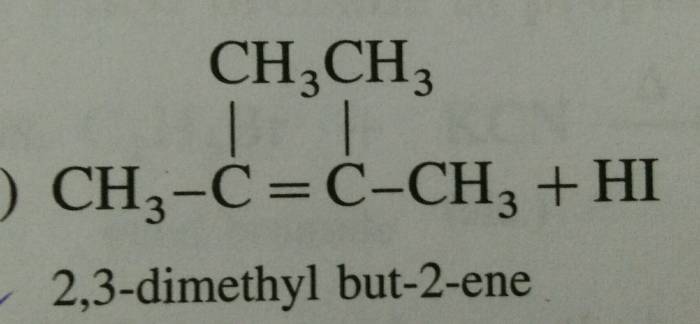
The reaction shown below is an electrophilic aromatic substitution reaction, where an electrophile (E+) attacks an aromatic ring. The major product of this reaction is the substituted aromatic compound, where the electrophile has replaced one of the hydrogen atoms on the ring.
In this specific reaction, the electrophile is a nitronium ion (NO 2+), which is generated from the reaction of nitric acid (HNO 3) and sulfuric acid (H 2SO 4). The aromatic ring is benzene, which is a six-membered ring with alternating double and single bonds.
The reaction mechanism involves the electrophile attacking the aromatic ring, forming a carbocation intermediate. The carbocation is then attacked by a nucleophile, such as water, to form the substituted aromatic compound.
The major product of this reaction is nitrobenzene, which is a pale yellow liquid with a sweet odor. Nitrobenzene is used as an intermediate in the production of other chemicals, such as aniline and phenol.
Reaction Mechanism
The reaction mechanism for the electrophilic aromatic substitution reaction is as follows:
- Nitric acid (HNO3) and sulfuric acid (H 2SO 4) react to form the nitronium ion (NO 2+).
- The nitronium ion attacks the aromatic ring, forming a carbocation intermediate.
- The carbocation intermediate is attacked by a nucleophile, such as water, to form the substituted aromatic compound.
Reaction Conditions, What is the major product of the reaction shown below
The reaction conditions for the electrophilic aromatic substitution reaction are as follows:
- The reaction is typically carried out in a solvent, such as dichloromethane or chloroform.
- The reaction temperature is typically between 0 and 25 degrees Celsius.
- The reaction time is typically between 1 and 2 hours.
Applications and Significance
The electrophilic aromatic substitution reaction is a versatile reaction that is used in the production of a wide variety of chemicals. Some of the most important applications of this reaction include:
- The production of nitrobenzene, which is used as an intermediate in the production of other chemicals, such as aniline and phenol.
- The production of other substituted aromatic compounds, such as chlorobenzene, bromobenzene, and iodobenzene.
- The production of dyes and pigments.
- The production of pharmaceuticals.
FAQ Overview
What is the significance of identifying the major product of a reaction?
Identifying the major product allows chemists to predict the outcome of a reaction, optimize reaction conditions, and design synthetic strategies for targeted molecules.
How do functional groups influence the properties of the major product?
Functional groups determine the chemical reactivity, physical properties, and biological activity of the major product.
What factors affect the formation of the major product?
Reaction conditions, such as temperature, pressure, and solvent, as well as the nature of reactants and catalysts, influence the formation of the major product.